When recognising a new species or subspecies, a “holotype” (i.e., the specimen from which the subspecies or species was first described) is required. The holotype for Calyptorhynchus banksii escondidus is a specimen held at CSIRO’s Australian National Wildlife Collection (ANWC), specimen ANWC B37847. (Image: Gordon Gullock, via Kyle Ewart.) Gordon Gullock.
GrrlScientist, Cockatoo Subspecies Identified In Australia, Forbes, 31 May 2020
Evolutionary & behavioural ecologist, ornithologist & science writer.
A large genetic study has uncovered a new subspecies of one of Australia’s most iconic birds, the red-tailed black cockatoo, Calyptorhynchus banksii. The newly identified subspecies is unique to inland Western Australia and lives in regions spanning the Wheatbelt, east of Perth, to the Pilbara in the state’s north-west.
“Escondidus is new — it’s the subspecies’ name and it basically means ‘hidden’ because it’s been hidden in plain sight”, said lead author of the study, conservation biologist Kyle Ewart, a PhD Candidate at the University of Sydney and a Research Associate at the Australian Museum’s Centre for Wildlife Genomics, where he researches the population genomics of two commonly traded Australian cockatoo species.
Mr Ewart said they knew the population was in the region but until now had assumed they were the same as the inland subspecies. But genetic testing revealed that the newly identified Western Australia red-tailed black cockatoo is more closely related to the forest red-tailed cockatoo than to the inland subspecies.
Red-tailed black cockatoos are one of Australia’s most iconic birds
The red-tailed black cockatoo is a large black parrot with scarlet panels in its long rounded tail that is hard to miss. Females and immatures can be readily identified by their black-striped orange tail panels, horn-colored beaks, and by the buff-colored stripes and polka-dots sprinkled throughout their black plumage.

Native to Australia, this seed-eating parrot ranges across much of the continent, and is quite common in eucalyptus or casuarina woodlands. Some subspecies are mainly found in Brown Stringybark forests in south-western Victoria and south-eastern South Australia, whilst others prefer Marri, Jarrah and Karri forests in south-western Australia. Yet other subspecies are less restricted in their habitat preferences, and even occur in several urban areas and regional towns.
Like other cockatoos, red-tailed black cockatoos cannot excavate their own tree hollows, yet they depend upon them for nesting. Further, the red-tailed black-cockatoo is also targeted by the illegal wildlife trade.
There are five recognized subspecies of red-tailed black cockatoos (Figure 1), all of which can be distinguished on the basis of body size, plumage color, beak size and geographic range. The two southern subspecies — the forest red-tailed black cockatoo, C. b. naso and the south-eastern red-tailed black cockatoo, C. b. graptogyne — have small ranges that are in or near urban areas. Currently, it is estimated that there are approximately 15,000 naso (Vulnerable) and only 1,000 graptogyne (Endangered) individuals remaining in the wild. Thus, genetic information is greatly needed to inform successful conservation strategies for the entire species.

Surprisingly, despite ongoing and widespread habitat destruction, there has not been a comprehensive genetic analysis of this species. As a result, it is difficult to design and implement informed conservation priorities and strategies, especially for the populations living in the northern tropical and arid regions of Australia.
So Mr Ewart and an international team of collaborators from the Australian Museum, the University of Sydney, The Commonwealth Scientific and Industrial Research Organisation (CSIRO), and the University of Edinburgh conducted a large comprehensive genomic assessment of red-tailed black cockatoos from across their entire range. The team collected samples from specimens stored for many decades in museums throughout Australia.
“We were able to extract a large amount of genetic data from these specimens, some of which were over 100 years old”, Mr Ewart said.

Mr Ewart and his collaborators used advanced DNA sequencing and analysis techniques to (1) investigate the relationships between currently identified subspecies and (2) to help them develop effective conservation strategies.
The red-tailed black cockatoos had several surprises in store
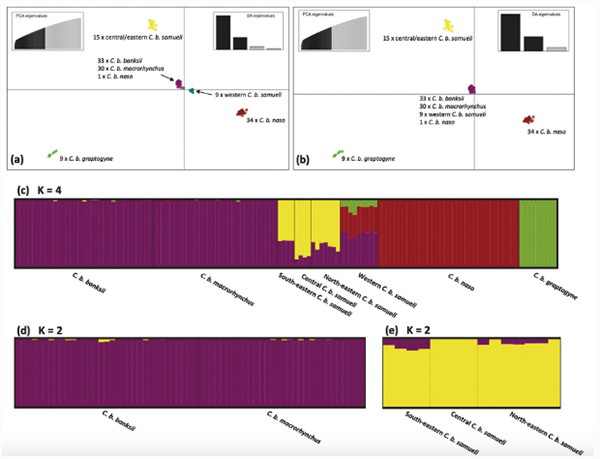
Based on the data, Mr Ewart and his collaborators estimated that the ancestor of red-tailed black cockatoos appeared within the past ~1.28 million years. They also identified five ‘conservation units’ (subspecies) within the species (Figure 2).
Mr Ewart and his collaborators also established that the Endangered graptogyne subspecies of western Victoria and south-eastern South Australia has the lowest overall level of genetic diversity, and is probably suffering from inbreeding.

Mr Ewart and his collaborators were surprised to discover that the ‘five conservation units’ identified by their DNA analyses were not the same five subspecies that had been recognized previously (Figure 4).
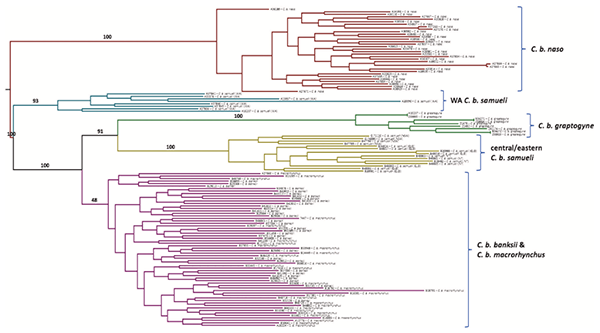
Based on the DNA data, Mr Ewart and his collaborators made two changes to the formal classification within the species. First, because they did not find any genetic separation between the two subspecies, banksii and macrorhynchus (Figure 4), found across northern Australia, these two northern subspecies were combined into a single subspecies, banksii. This is despite the fact that the sparsely wooded plains fringing the Gulf of Carpentaria are known to physically separate many species in this region, but it apparently does not form ay sort of a barrier between banksii and macrorhynchus (Figure 4).
Looks versus genetics: similar looks are not necessarily closest relatives
But what was most surprising about this study?
“The discovery of this new subspecies surprised me most”, Mr Ewart said in email.
“Before this research, this new subspecies was considered part of the inland red-tailed black-cockatoo subspecies. We thought it might be genetically different due to its geographic isolation to the other inlaid populations, but we didn’t expect it to be that different. We found it was more closely related to the forest subspecies.”
Thus, the second taxonomic change that Mr Ewart and his collaborators made was to assign the samueli populations living east of Perth and on the Wheatbelt in Western Australian to a new subspecies. The genetic data showed these populations were most closely related to the forest red-tailed black-cockatoo, naso, and not to the other populations of samueli scattered across Australia.
How did this newly identified subspecies arise in the apparent absence of a physical barrier?
“This newly discovered subspecies, Calyptorhynchus banksii escondidus, likely evolved and diverged from the inland and northern subspecies in the presence of the vast deserts of Western Australia, and from the south-western subspecies (i.e. C. b. naso, the forest red-tailed black-cockatoo) in the presence of tracts of light woodlands”, Mr Ewart elaborated. “However, C. b. escondidus seems to have expanded its range more recently due to the emergence of agriculture in this region.”
Mr Ewart and his collaborators named the new subspecies escondidus, meaning ‘hidden’ in Spanish, because for many decades, it was ‘hidden in plain sight’ as part of samueli.
Although they are not each other’s closest relatives, the physical similarities between escondidus and samueli are probably the result of convergent evolution, a process where they ended up looking alike because they live in similar habitats and have similar ecologies, despite having different genetics.
“The ecology of C. b. escondidus appears to be key to morphological differences between it and the forest red-tailed-black cockatoo subspecies, its closest relative”, Mr Ewart noted in email.
“C. b. escondidus is a ground feeder, whereas the forest subspecies is mostly arboreal. The inland red-tailed black cockatoos are also ground feeders, thus similarities between between C. b. escondidus and the inland subspecies are likely to be due to similar feeding ecologies.”
“They evolved to be similar because they both feed on the ground and live in similar arid and semi-arid habitats”, Mr Ewart summarized.
And yet despite their many physical similarities, avian morphologist and co-author of this study, Richard Schodde, identified specific distinguishing features between escondidus and samueli.
This research would not be possible without museum specimens
Extensive museum collections are the only way that large collaborative studies like this are possible. For example, in addition to samples from the Australian Museum, more samples were provided by the Australian National Wildlife Collection, the Western Australian Museum and Museums Victoria.
“It only took decades of Museum collections and an extensive genetic analysis to recognise them!”
Specimens were added to these museums’ collections throughout the decades, long before anyone knew about or could imagined the power of cutting-edge DNA technologies. As a result, not only has a new subspecies of red-tailed black cockatoo been identified, but this genetic data will help conservation biologists outline a strategy to help rescue the Endangered graptogyne subspecies from continued loss of genetic diversity.
“We hope that this study can be used to inform conservation strategies to ensure the ongoing survival of this magnificent species.”
Source:
Kyle M. Ewart, Nathan Lo, Rob Ogden, Leo Joseph, Simon Y. W. Ho, Greta J. Frankham, Mark D. B. Eldridge, Richard Schodde and Rebecca N. Johnson (2020). Phylogeography of the iconic Australian red-tailed black-cockatoo (Calyptorhynchus banksii) and implications for its conservation, Heredity, published online on 12 May 2020 ahead of print | doi:10.1038/s41437-020-0315-y

